Quillivant XR® delivers
Eligible patients may PAY $25
Learn more about Savings & SupportStart with a low dose and titrate to balance efficacy and side effects

Quillivant XR is taken once daily
in the morning, with or without food
- Uses innovative LiquiXR® technology
- Liquid formulation available in 4 volume sizes
- With the calibrated oral dosing dispenser, the dose may be adjusted to meet the individual treatment needs of the patient
— Because Quillivant XR (methylphenidate HCl) can be titrated in small increments, you can work with your patients to determine the right dose
Patient assessment
- Prior to treating patients with Quillivant XR, assess for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical examination) and risk for abuse, misuse, and addiction. Assess the family history and clinically evaluate patients for motor or verbal tics or Tourette's syndrome before initiating Quillivant XR
- If paradoxical aggravation of symptoms or other adverse reactions occur, reduce dosage or discontinue the drug, if necessary. If improvement is not observed after appropriate dosage adjustment over a 1-month period, the drug should be discontinued
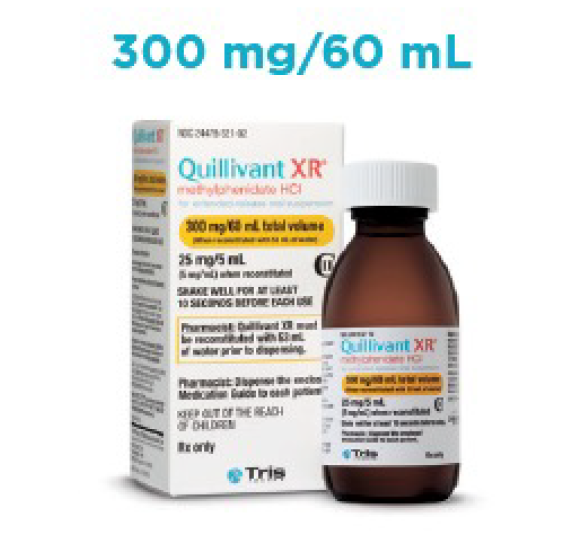
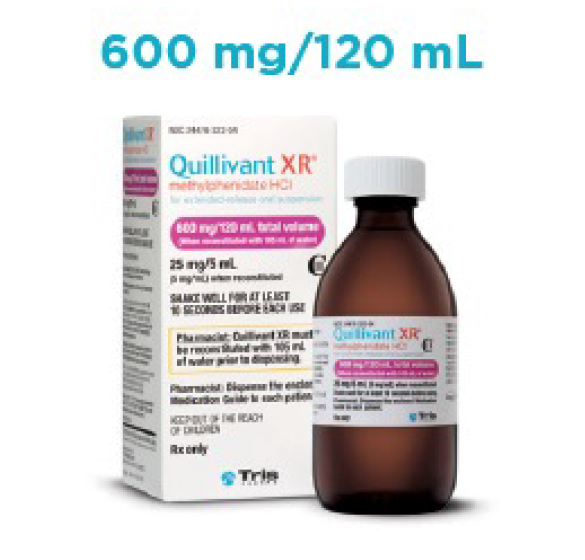
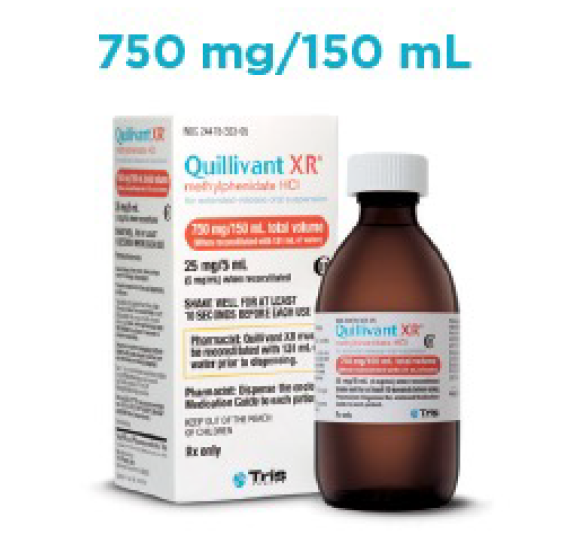
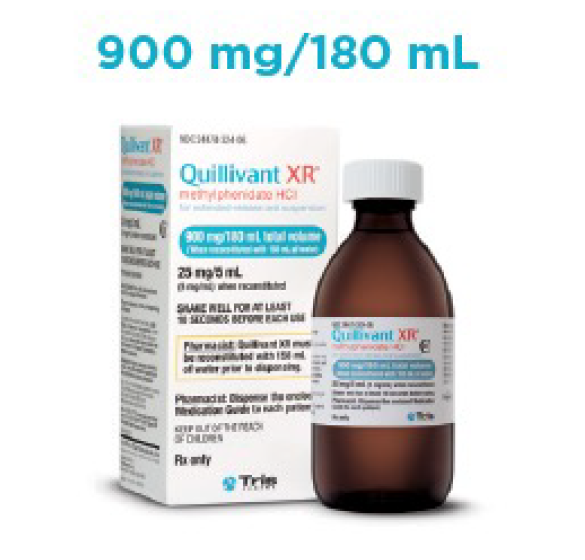
Quillivant XR Prescribing Guide

Formulation and administration
- Quillivant XR must be reconstituted with water by a pharmacist
- After reconstitution, the pharmacist must fully insert the bottle adapter into the neck of the bottle
- Before administering each dose, instruct the patient/caregiver to vigorously shake the bottle of Quillivant XR for at least 10 seconds to prepare suspension
Important reminders for pharmacists
- Quillivant XR is supplied as a powder for oral suspension which must be reconstituted with water prior to dispensing
- After adding the specified amount of water to the bottle, be sure to fully insert bottle adapter into neck of bottle
- Replace bottle cap. Shake with vigorous back and forth motion for at least 10 seconds to prepare suspension before dispensing prescription
- See section 2.6 in Full Prescribing Information for additional details on reconstitution
If switching from other methylphenidate products to Quillivant XR:
- Discontinue that treatment and titrate with Quillivant XR using the above titration schedule
- Do not substitute for other methylphenidate products on a milligram-per-milligram basis because of different methylphenidate base compositions and differing pharmacokinetic profiles
Writing a prescription for Quillivant XR
Example prescription

When prescribing multiple bottle volumes of Quillivant XR with different NDCs when 1 bottle could be used to fill the prescription, it is important to remember:
- This may result in additional out-of-pocket or co-pay costs for your patients
- Insurance may cover a prescription for multiple bottle volumes within one 30-day time frame; however, this may require prior authorization or a call to your patient's insurance company
CNS, central nervous system; NDC, National Drug Code; PO, by mouth.
References: 1. Quillivant XR [package insert]. Tris Pharma, Inc., Monmouth Junction, NJ. 2. Wigal SB, Childress AC, Belden HW, Berry SA. J Child Adolesc Psychopharmacol. 2013;23(1):3-10.


